Navigating the Medical Device Regulatory Affairs Market: Key Success Factors and Beyond
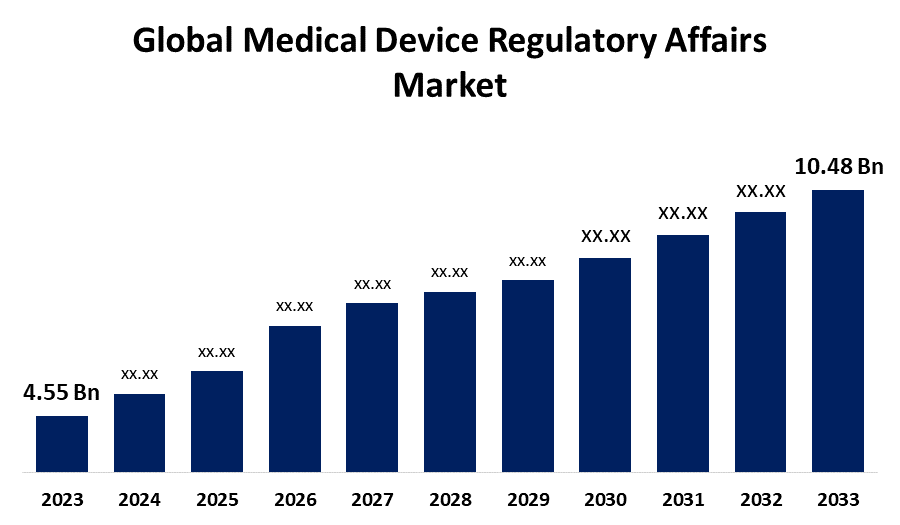
According to a research report published by Spherical Insights & Consulting, the Global Medical Device Regulatory Affairs Market Size is Expected to Grow from USD 4.55 Billion in 2023 to USD 10.48 Billion by 2033, Growing at a CAGR of 8.70 % during the forecast period 2023-2033.
Get a Sample PDF Brochure: https://www.sphericalinsights.com/request-sample/5985
The field of medical device regulatory affairs emerged from the government’s desire to protect public health by regulating the effectiveness and safety of goods produced by medical device manufacturers. Additionally, the growing need for expedited clearance procedures and the changing regulatory environment will propel market expansion over the forecast period. The market is also developing as a result of consumer demand for innovative technology-based medical devices, medical device companies increased outsourcing investments, a growing pipeline of medical devices, and supportive government policies. Furthermore, market participants strive for quicker product approvals to increase their market share, as regulatory approval in the medical device sector becomes more rigorous and time-consuming. As a result, medical device manufacturers must constantly adapt to shifting regulatory standards across a range of industries and regions. However, the global expansion of the medical device regulatory affairs market would be hampered by a number of factors. Strict restrictions can be costly and time-consuming to comply with, which can delay product development and raise costs.
Browse key industry insights spread across 240 pages with 120 Market data tables and figures & charts from the report on the Global Medical Device Regulatory Affairs Market Size, Share, and COVID-19 Impact Analysis, By Regulatory Phase (Pre-Market and Post-Market), By Service (Product Registration & Clinical Trial Applications, Regulatory Consulting, Legal and Representation, and Regulatory Writing & Publishing) By Type (Therapeutics and Diagnostic), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033.
Buy Now Full Report: https://www.sphericalinsights.com/checkout/5985
The post-market segment is expected to hold the largest share of the global medical device regulatory affairs market during the forecast period.
Based on the regulatory phase, the medical device regulatory affairs market is classified into pre-market and post-market. Among these, the post-market segment is expected to hold the largest share of the market during the forecast period. The need for specialist post-market regulatory services is increasing as a result of companies entering international markets having to adhere to many post-market norms in numerous jurisdictions. Additionally, the post-market segment is expanding due to the increased focus on protecting patients’ safety and health throughout the medical device’s life cycle.
The regulatory consultant segment is expected to grow at the fastest CAGR in the global medical device regulatory affairs market during the forecast period.
Based on the services, the medical device regulatory affairs market is divided into product registration & clinical trial applications, regulatory consulting, legal representation, and regulatory writing & publishing. Among these, the regulatory consultant segment is expected to grow at the fastest CAGR in the market during the forecast period. Regulatory consultants assist businesses in adhering to several categories of continuously changing standards by providing comprehensive assessments and strategic guidance.
The therapeutic segment is expected to grow at the fastest CAGR in the global medical device regulatory affairs market during the forecast period.
Based on the type, the medical device regulatory affairs market is categorized into therapeutics and diagnostic. Among these, the therapeutic segment is expected to grow at the fastest CAGR in the market during the forecast period. Raises attention to the need for regulatory support to launch novel therapeutic devices. Significant funds are also allocated to the study and creation of novel medicinal devices, particularly in fields such as neurology, cardiology, and oncology.
Inquire Before Buying This Research Report: https://www.sphericalinsights.com/inquiry-before-buying/5985
North America is estimated to hold the largest share of the medical device regulatory affairs market over the forecast period.
North America is estimated to hold the largest share of the medical device regulatory affairs market over the forecast period. The market is expanding as a result of the large number of medical device businesses operating in the United States, which are pursuing regulatory affairs and seeking advice for their product registration, clinical trial application, and legal representation. The regional market is also anticipated to rise as a result of heightened government and regulatory agency efforts to prevent medical device failure and harmful impacts. Furthermore, the need for more inventive medical gadgets has grown as a result of the nation’s updated healthcare system. It is anticipated that this will increase market demand for a range of medical devices, increasing the need for medical device regulatory affairs.
Asia Pacific is predicted to have the highest CAGR growth in the medical device regulatory affairs market over the forecast period. Rapidly expanding economies, such as China and India, are investing heavily in healthcare technology and infrastructure, which raises the demand for medical equipment and regulatory support services. Additionally, medical device manufacturers are drawn to the Asia Pacific region due to its low manufacturing costs. Furthermore, the regional market is expanding due to the aging population, the incidence of chronic illnesses, and the government’s growing support for the healthcare industry. Because of this, it is expected that more people would enter the market, which will boost regional market expansion.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market.Major key players in the Market include TUV SUD, SGS, UL (Underwriters Laboratories), Bureau Veritas, Medpace, PAREXEL, NAMSA, Charles River, Regulatory Compliance Associates, RAPS (Regulatory Affairs Professionals Society), CRB, HCL Technologies, Eurofins Scientific, Catalent, Celerion, and Others.
Recent Developments
- In June 2024, IMed Consultancy launched a new white paper assessing the regulatory state for Artificial Intelligence (AI) & Machine Learning (ML)-powered medical devices in the U.S., UK, and EU.
Key Target Audience
- Market Players
- Investors
- End-users
- Government Authorities
- Consulting And Research Firm
- Venture capitalists
- Value-Added Resellers (VARs)
Market Segment
This study forecasts revenue at global, regional, and country levels from 2023 to 2033. Spherical Insights has segmented the medical device regulatory affairs market based on the below-mentioned segments:
By Regulatory Phase
- Pre-Market
- Post-Market
By Service
- Product Registration & Clinical Trial Applications
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
By Type
- Therapeutics
- Diagnostic
By Regional Analysis
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company’s mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us